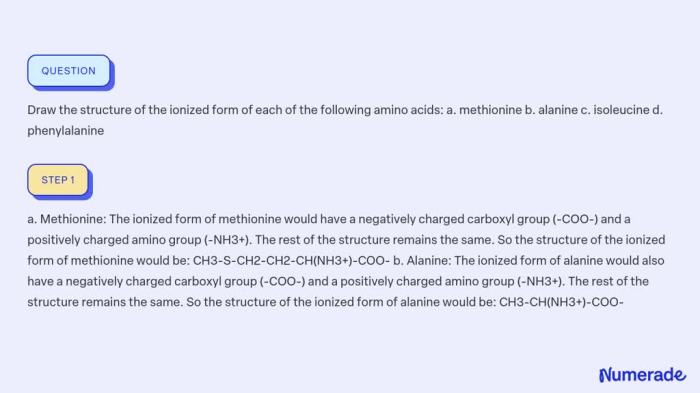
Write the structure of the ionized form of phenylalanine, an exploration into the chemical intricacies of this essential amino acid. This discourse delves into the ionization process, properties, biological significance, and diverse applications of ionized phenylalanine, unraveling its multifaceted nature.
Ionized phenylalanine, a derivative of the ubiquitous amino acid phenylalanine, possesses a unique chemical structure and set of properties that distinguish it from its neutral counterpart. Understanding the intricacies of its ionized form is crucial for comprehending its behavior in various chemical and biological contexts.
Ionized Form of Phenylalanine: Write The Structure Of The Ionized Form Of Phenylalanine
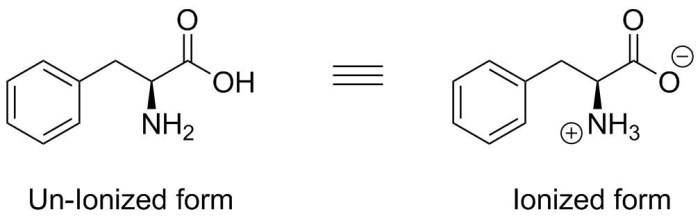
Phenylalanine is an essential amino acid with a chemical structure that can be ionized in specific conditions. Ionization refers to the process of gaining or losing electrons, resulting in the formation of charged species. The ionized form of phenylalanine, denoted as Phe –, is an important intermediate in various biological processes and has unique properties compared to its neutral form.
Chemical Structure, Write the structure of the ionized form of phenylalanine
The chemical structure of the ionized form of phenylalanine differs from its neutral form by the presence of an additional negative charge. This negative charge is localized on the carboxyl group (-COOH) of phenylalanine, which loses a proton (H +) during ionization.
The resulting structure of Phe –is a zwitterion, meaning it contains both positive and negative charges within the same molecule.
Process of Ionization
Ionization of phenylalanine occurs when it is exposed to an alkaline environment, such as a solution with a high pH. In such conditions, the carboxyl group of phenylalanine is deprotonated, releasing a proton and forming Phe –. The ionization process is reversible, and the equilibrium between the neutral and ionized forms of phenylalanine depends on the pH of the solution.
Properties of Ionized Phenylalanine
The ionized form of phenylalanine exhibits distinct physical and chemical properties compared to its neutral form. These properties include:
- Solubility:Phe –is more soluble in water than its neutral form due to its increased polarity. The negative charge on the carboxyl group enhances its interaction with water molecules, making it more hydrophilic.
- Reactivity:The ionized form of phenylalanine is more reactive than its neutral form. The negative charge on the carboxyl group makes it a better nucleophile, which can participate in various chemical reactions more readily.
- pH Dependence:The ionization of phenylalanine is pH-dependent. At low pH, the neutral form predominates, while at high pH, the ionized form becomes more prevalent. This pH dependence is important for the biological function of phenylalanine in different cellular compartments.
Biological Significance of Ionized Phenylalanine
The ionized form of phenylalanine plays a crucial role in various biological systems. Its unique properties allow it to participate in essential cellular processes, including:
- Protein Synthesis:Ionized phenylalanine is incorporated into proteins during protein synthesis. It is one of the 20 essential amino acids required for the synthesis of various proteins in the body.
- Metabolic Pathways:Phe –is involved in several metabolic pathways, such as the phenylalanine hydroxylase pathway. This pathway is responsible for the conversion of phenylalanine to tyrosine, another essential amino acid.
- Ion Transport:The ionized form of phenylalanine can facilitate the transport of ions across biological membranes. Its negative charge allows it to interact with positively charged ions, enabling their movement across the membrane.
Applications of Ionized Phenylalanine
The unique properties of ionized phenylalanine have led to its potential applications in various fields, including:
- Pharmaceuticals:Phe –can be used as a precursor for the synthesis of drugs and pharmaceutical compounds. Its high reactivity and solubility make it a suitable starting material for various chemical reactions.
- Food Industry:Ionized phenylalanine can be used as a food additive to enhance the flavor and nutritional value of food products. Its ability to interact with other molecules can contribute to the formation of desirable flavors and textures.
- Research and Development:Phe –is used in scientific research to study the structure and function of proteins. Its unique properties make it a valuable tool for understanding the molecular basis of biological processes.
FAQ Explained
What is the chemical structure of ionized phenylalanine?
Ionized phenylalanine carries a net negative charge, with its carboxyl group (-COOH) deprotonated to (-COO-) and its amino group (-NH2) protonated to (-NH3+).
How does phenylalanine become ionized?
Phenylalanine undergoes ionization when it loses a proton (H+) from its carboxyl group, resulting in the formation of the ionized form.
What are the key properties of ionized phenylalanine?
Ionized phenylalanine exhibits altered solubility, reactivity, and other characteristics compared to its neutral form, influencing its behavior in different environments.
What is the biological significance of ionized phenylalanine?
Ionized phenylalanine plays crucial roles in various biological processes, including protein synthesis, enzyme catalysis, and cellular signaling.
What are the potential applications of ionized phenylalanine?
Ionized phenylalanine finds applications in diverse fields, such as food additives, pharmaceutical formulations, and industrial processes, owing to its unique properties.