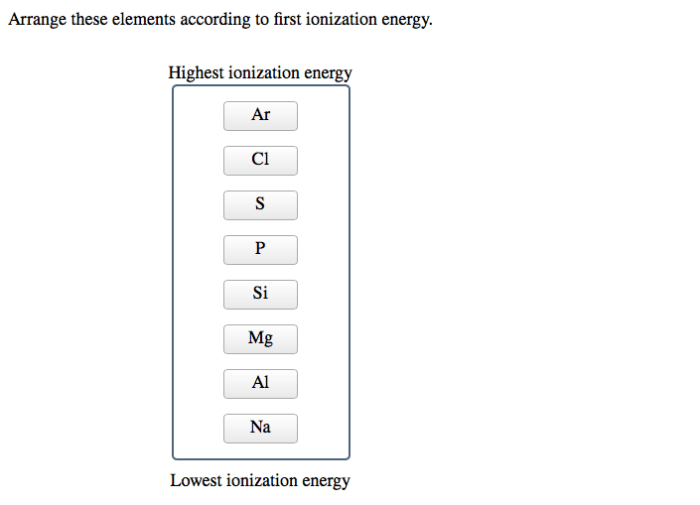
Arrange the elements according to first ionization energy – Delving into the fascinating realm of atomic structure, we embark on a journey to understand the concept of first ionization energy and its profound implications. This comprehensive guide will delve into the factors that influence this crucial property, explore the periodic trends it exhibits, and uncover its diverse applications across scientific disciplines.
By unraveling the intricate relationship between an element’s electronic configuration and its first ionization energy, we gain invaluable insights into the behavior of matter at the atomic level.
1. Introduction
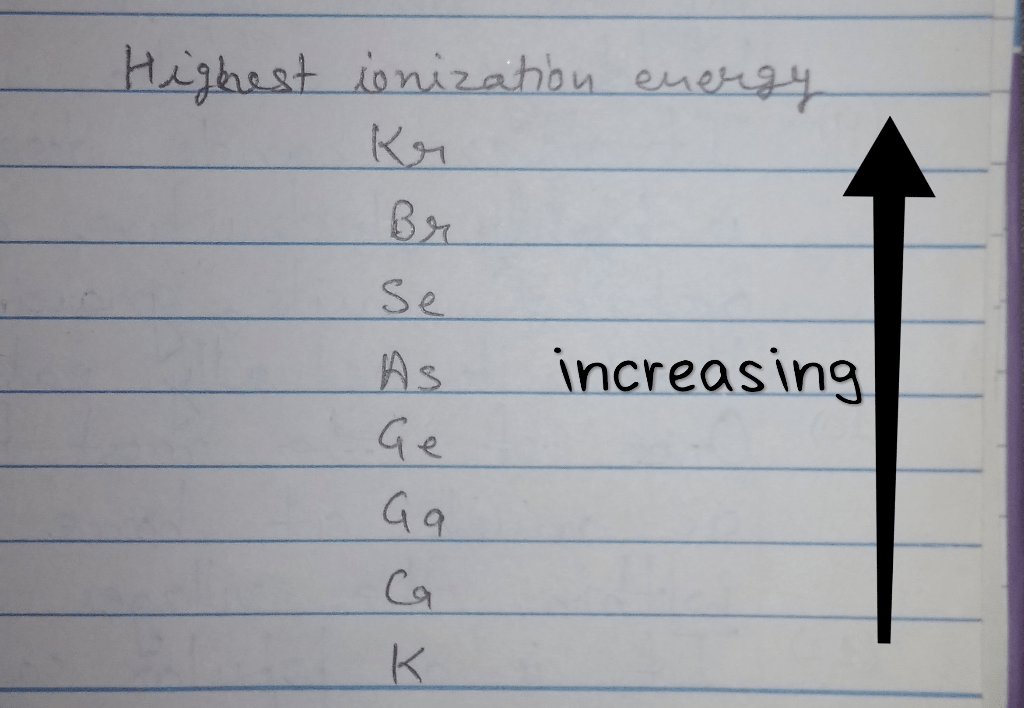
Ionization energy is the minimum amount of energy required to remove an electron from an atom or ion in its gaseous state. Understanding the first ionization energy of elements is crucial in chemistry, physics, and materials science.
The first ionization energy provides insights into the reactivity, stability, and electronic structure of elements. It influences various chemical processes, including bond formation, redox reactions, and the formation of ions and radicals.
2. Factors Affecting First Ionization Energy
Several factors influence the first ionization energy of elements:
2.1. Atomic Radius
The atomic radius, which is the distance from the nucleus to the outermost electron shell, plays a significant role. Generally, as the atomic radius increases, the first ionization energy decreases. This is because the outermost electron is farther from the nucleus, making it easier to remove.
2.2. Nuclear Charge
The nuclear charge, or the number of protons in the nucleus, also affects the first ionization energy. As the nuclear charge increases, the attraction between the nucleus and the outermost electron increases, making it harder to remove and resulting in a higher first ionization energy.
2.3. Electron Configuration, Arrange the elements according to first ionization energy
The electron configuration, particularly the number of electrons in the outermost shell, influences the first ionization energy. Elements with a half-filled or completely filled outermost shell (i.e., noble gases) have higher first ionization energies due to their stable electron configurations.
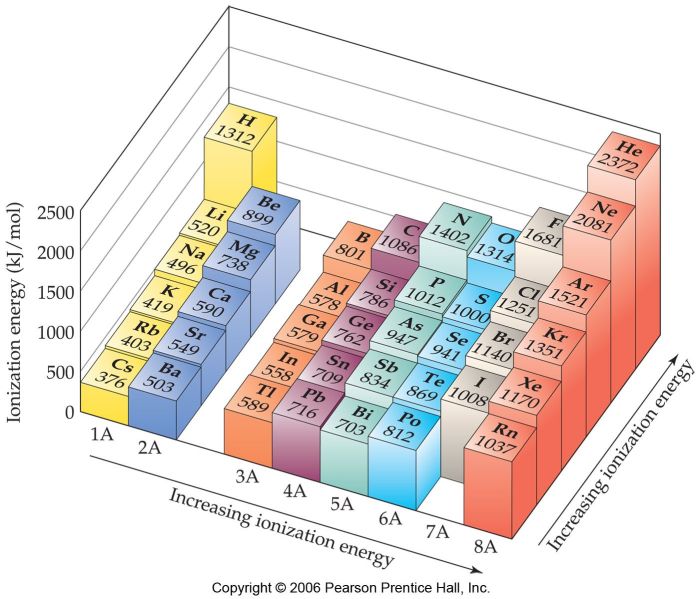
3. Trends in First Ionization Energy
The first ionization energy exhibits periodic trends across the periodic table:
3.1. Group Trends
Within a group (vertical column), the first ionization energy generally decreases down the group. This is because the atomic radius increases down the group, making it easier to remove the outermost electron.
3.2. Period Trends
Across a period (horizontal row), the first ionization energy generally increases from left to right. This is because the nuclear charge increases from left to right, making it harder to remove the outermost electron.
4. Applications of First Ionization Energy
The first ionization energy has various applications in different fields:
4.1. Chemistry
- Predicting the reactivity of elements
- Understanding bond formation and stability
- Determining the oxidation states of elements
4.2. Physics
- Calculating the energy required for ionization processes
- Studying the electronic structure of atoms and ions
- Developing new materials and technologies
4.3. Materials Science
- Designing materials with specific electronic properties
- Improving the efficiency of electronic devices
- Developing new energy storage and conversion technologies
5. Exceptions and Anomalies: Arrange The Elements According To First Ionization Energy
There are some exceptions and anomalies to the general trends in first ionization energy:
- Potassium (K)has a lower first ionization energy than calcium (Ca), despite having a higher nuclear charge. This is due to the presence of an extra electron in the 4s orbital of potassium, which makes it easier to remove.
- Chromium (Cr)has a higher first ionization energy than manganese (Mn), even though manganese has a higher nuclear charge. This is because chromium has a stable half-filled 3d orbital, which makes it harder to remove an electron.
FAQ Compilation
What is the significance of first ionization energy?
First ionization energy is a fundamental property that governs the chemical reactivity and physical behavior of elements. It provides insights into the ease with which an atom can lose an electron, influencing its bonding capabilities, reactivity, and overall chemical properties.
How does atomic radius affect first ionization energy?
As atomic radius increases, the distance between the nucleus and outermost electron increases, resulting in a decrease in the electrostatic attraction between them. This weaker attraction makes it easier to remove the electron, leading to a lower first ionization energy.
What is the relationship between electron configuration and first ionization energy?
Electron configuration plays a crucial role in determining first ionization energy. Elements with more stable electron configurations, such as noble gases, have higher first ionization energies due to the strong electrostatic attraction between the nucleus and the tightly bound electrons.
