Water vapor is cooled in a closed rigid tank from an initial state of high temperature and pressure. As the cooling process progresses, the water vapor undergoes significant changes in its thermodynamic properties, including pressure, temperature, and volume. This article explores the intricate details of this cooling process, examining the governing equations, energy transfer mechanisms, and phase change phenomena involved.
The cooling of water vapor in a closed rigid tank finds applications in various industries, including power generation, refrigeration, and chemical processing. Understanding the underlying principles of this process is crucial for optimizing its performance and efficiency in these applications.
Cooling Water Vapor in a Closed Rigid Tank
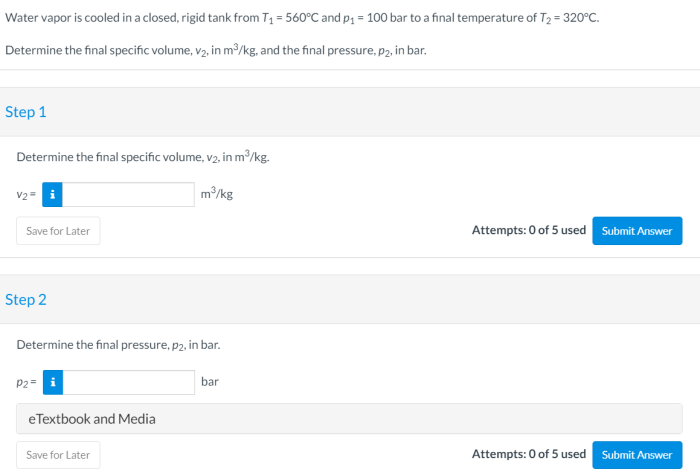
Cooling water vapor in a closed rigid tank is a process that involves reducing the temperature of water vapor while maintaining a constant volume. This process finds applications in various industries, including power generation, chemical processing, and refrigeration.
Process Description
Initially, water vapor is contained within a closed rigid tank at a certain temperature and pressure. To cool the water vapor, heat is removed from the tank through its walls or by other means, such as a cooling coil. As heat is removed, the water vapor undergoes a decrease in temperature.
During cooling, the pressure of the water vapor also decreases. This is because the volume of the tank is constant, and as the temperature decreases, the molecular motion of the water vapor slows down, resulting in a reduction in pressure.
Thermodynamic Properties
The equation of state for water vapor in a closed rigid tank is given by the ideal gas law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of water vapor, R is the ideal gas constant, and T is the temperature.
The specific heat of water vapor is a measure of the amount of heat required to raise the temperature of one unit mass of water vapor by one degree Celsius. The specific heat of water vapor varies with temperature and pressure.
Energy Transfer
Heat is transferred from the water vapor to the surroundings through the walls of the tank by conduction and convection. Conduction is the transfer of heat through direct contact between two substances, while convection is the transfer of heat through the movement of a fluid.
The thermal conductivity of the tank walls and the surrounding fluid determine the rate of heat transfer. A higher thermal conductivity facilitates faster heat transfer.
Condensation and Phase Change, Water vapor is cooled in a closed rigid tank from
As the water vapor is cooled, it reaches a point where it condenses into liquid water. Condensation occurs when the temperature and pressure of the water vapor reach the saturation point, where the vapor pressure equals the vapor pressure of liquid water at the same temperature.
During condensation, the water vapor undergoes a phase change from a gas to a liquid. This phase change is accompanied by the release of latent heat, which is the heat that was absorbed by the water vapor during evaporation.
Applications
Cooling water vapor in a closed rigid tank has various applications, including:
- Power generation: In steam power plants, water vapor is cooled in condensers to convert it back into liquid water, which is then recycled to the boiler.
- Chemical processing: In chemical plants, water vapor is cooled to condense and separate it from other gases or vapors.
- Refrigeration: In refrigeration systems, water vapor is cooled in evaporators to absorb heat from the surrounding environment.
FAQs: Water Vapor Is Cooled In A Closed Rigid Tank From
What is the initial state of water vapor in a closed rigid tank?
The initial state of water vapor in a closed rigid tank is characterized by high temperature and pressure.
What are the changes in pressure and temperature during cooling?
As the water vapor is cooled, both pressure and temperature decrease. The rate of decrease depends on the specific heat of water vapor and the heat transfer mechanisms involved.
What is the equation of state for water vapor in a closed rigid tank?
The equation of state for water vapor in a closed rigid tank is the ideal gas law, which relates pressure, temperature, and volume.
What are the modes of heat transfer involved in cooling water vapor?
The modes of heat transfer involved in cooling water vapor include conduction, convection, and radiation.
What are the effects of condensation on pressure, temperature, and volume?
Condensation leads to a decrease in pressure, temperature, and volume of the water vapor.