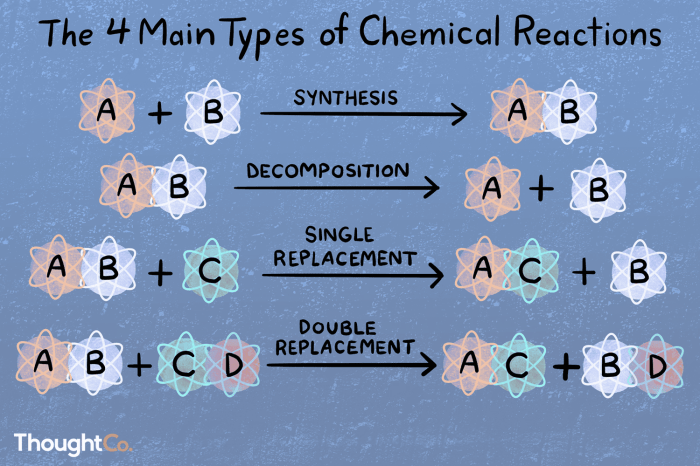
Five types of chemical reaction worksheet introduces the captivating realm of chemical reactions, where elements and compounds engage in intricate transformations, revealing the fundamental principles that govern the interactions of matter.
This comprehensive guide delves into the five primary types of chemical reactions, unveiling their unique characteristics, real-world applications, and the fascinating interplay of elements as they undergo chemical transformations.
Types of Chemical Reactions: Five Types Of Chemical Reaction Worksheet
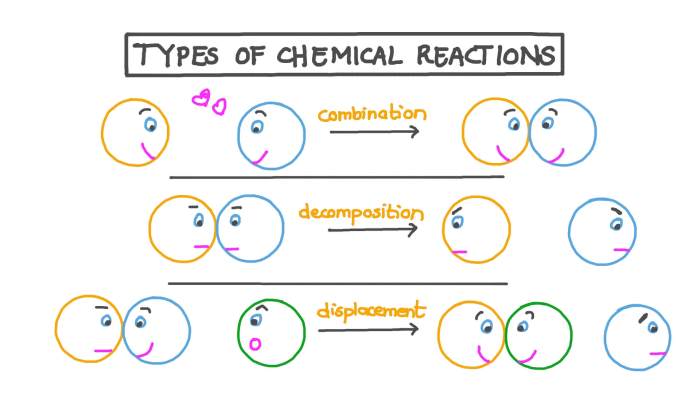
Chemical reactions are processes that involve the transformation of one set of substances into another. There are five main types of chemical reactions: synthesis, decomposition, single-replacement, double-replacement, and combustion reactions.
The following table summarizes the five types of reactions, their characteristics, and examples:
| Type of Reaction | Characteristics | Example |
|---|---|---|
| Synthesis | Two or more substances combine to form a new substance | 2H2 + O2 → 2H2O |
| Decomposition | A single substance breaks down into two or more simpler substances | 2H2O → 2H2 + O2 |
| Single-Replacement | One element replaces another element in a compound | Fe + 2HCl → FeCl2 + H2 |
| Double-Replacement | Ions from two ionic compounds exchange places | NaCl + AgNO3 → AgCl + NaNO3 |
| Combustion | A substance reacts with oxygen, releasing heat and light | CH4 + 2O2 → CO2 + 2H2O |
Synthesis Reactions
Synthesis reactions are chemical reactions in which two or more substances combine to form a new substance. The new substance is typically more complex than the original substances.
Examples of synthesis reactions include:
- The formation of water from hydrogen and oxygen
- The formation of carbon dioxide from carbon and oxygen
- The formation of sodium chloride from sodium and chlorine
Decomposition Reactions
Decomposition reactions are chemical reactions in which a single substance breaks down into two or more simpler substances. The original substance is typically more complex than the products.
Examples of decomposition reactions include:
- The decomposition of water into hydrogen and oxygen
- The decomposition of carbon dioxide into carbon and oxygen
- The decomposition of sodium chloride into sodium and chlorine
Single-Replacement Reactions
Single-replacement reactions are chemical reactions in which one element replaces another element in a compound. The element that is replaced is typically less reactive than the element that replaces it.
Examples of single-replacement reactions include:
- The reaction of iron with copper sulfate to form iron sulfate and copper
- The reaction of zinc with hydrochloric acid to form zinc chloride and hydrogen
- The reaction of magnesium with oxygen to form magnesium oxide
Double-Replacement Reactions, Five types of chemical reaction worksheet
Double-replacement reactions are chemical reactions in which ions from two ionic compounds exchange places. The products of a double-replacement reaction are typically two new ionic compounds.
Examples of double-replacement reactions include:
- The reaction of sodium chloride with silver nitrate to form silver chloride and sodium nitrate
- The reaction of potassium iodide with lead nitrate to form lead iodide and potassium nitrate
- The reaction of calcium chloride with sodium carbonate to form calcium carbonate and sodium chloride
Combustion Reactions
Combustion reactions are chemical reactions in which a substance reacts with oxygen, releasing heat and light. The substance that is burned is typically a hydrocarbon, such as methane, propane, or gasoline.
Examples of combustion reactions include:
- The burning of wood
- The burning of natural gas
- The burning of gasoline
FAQ Resource
What are the five main types of chemical reactions?
The five main types of chemical reactions are synthesis, decomposition, single-replacement, double-replacement, and combustion reactions.
What is the difference between a synthesis and a decomposition reaction?
In a synthesis reaction, two or more substances combine to form a new, more complex substance. In a decomposition reaction, a single substance breaks down into two or more simpler substances.
What is the purpose of a single-replacement reaction?
Single-replacement reactions are used to replace one element in a compound with another element.
What is the significance of double-replacement reactions?
Double-replacement reactions are important for the formation of precipitates, which are insoluble solids that form when two ionic compounds are mixed.
What is the role of combustion reactions in everyday life?
Combustion reactions are essential for many everyday processes, such as burning fuels to generate heat and power.