Embarking on a kinetic and thermal energy quick check, we delve into the fascinating world of energy, exploring its forms and transformations. Kinetic energy, the energy of motion, and thermal energy, the energy associated with temperature, play crucial roles in our everyday lives and in the vast tapestry of the universe.
In this comprehensive overview, we will unravel the concepts of kinetic and thermal energy, examining their characteristics, interrelationships, and practical applications. Prepare to be enlightened as we uncover the intricate dance of energy in our world.
Kinetic Energy: Kinetic And Thermal Energy Quick Check
Kinetic energy is the energy an object possesses due to its motion. It depends on the object’s mass and speed. Objects with higher mass or speed have greater kinetic energy.
Kinetic energy is present in various everyday situations. For instance, a moving car, a rolling ball, and a flying bird all possess kinetic energy.
Factors Affecting Kinetic Energy, Kinetic and thermal energy quick check
- Mass: The greater the mass of an object, the higher its kinetic energy at a given speed.
- Speed: The faster an object moves, the greater its kinetic energy for a given mass.
Thermal Energy
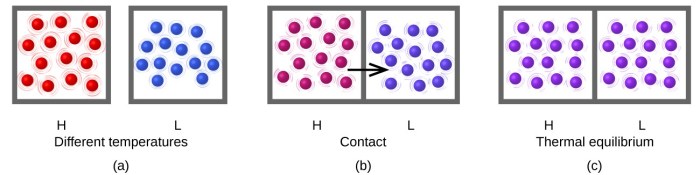
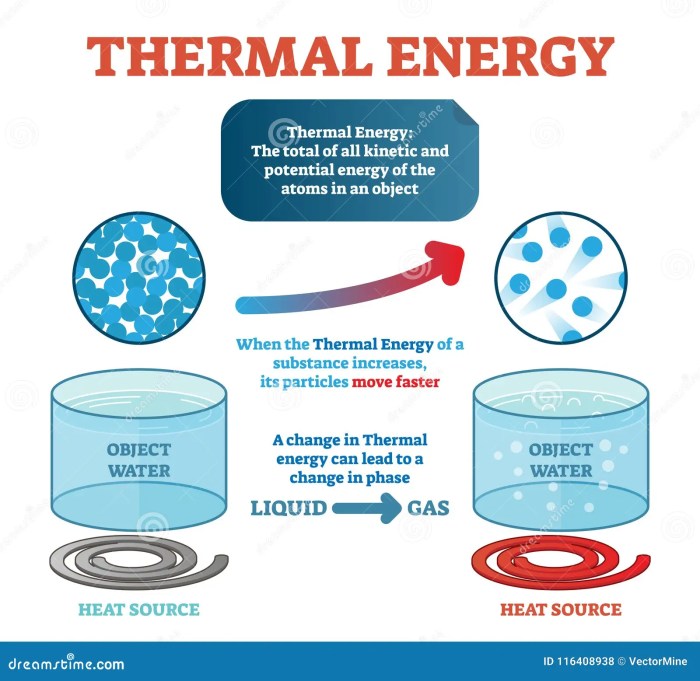
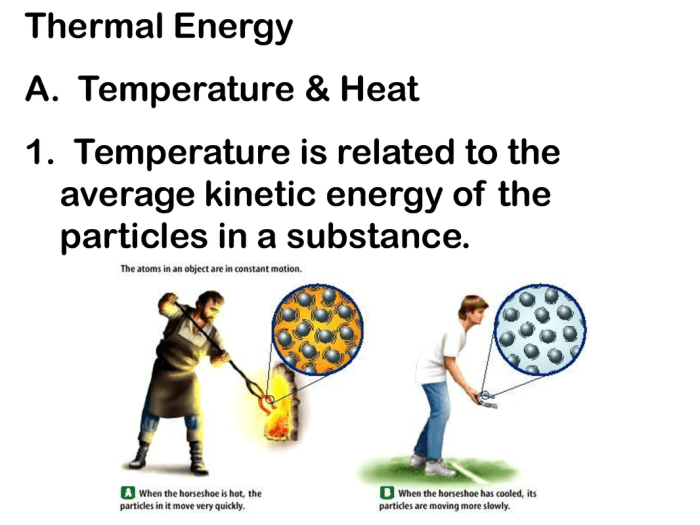
Thermal energy, also known as heat, is the energy associated with the random motion of particles within a substance. It is a measure of the temperature of a substance, where higher temperatures indicate greater thermal energy.
Transfer of Thermal Energy
Thermal energy can be transferred in three primary ways:
- Conduction: Heat transfer through direct contact between substances.
- Convection: Heat transfer through the movement of fluids (liquids or gases).
- Radiation: Heat transfer through electromagnetic waves, not requiring a medium.
Relationship between Kinetic and Thermal Energy
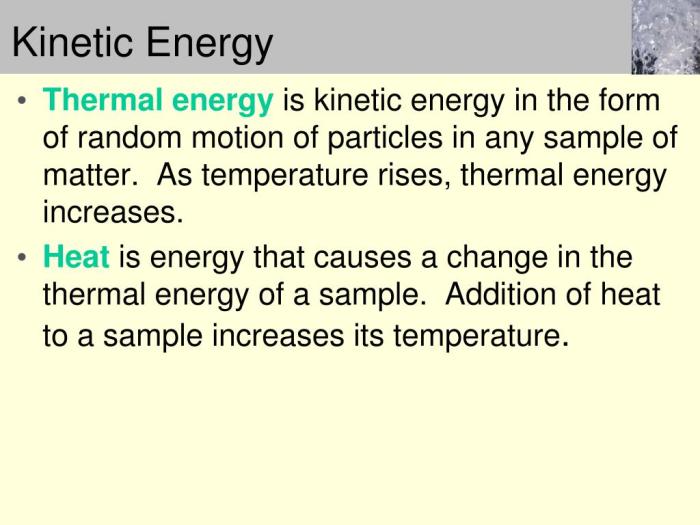
Kinetic energy and thermal energy are closely related. When an object is in motion, its kinetic energy can be converted into thermal energy. This conversion occurs due to friction or collisions between particles, generating heat.
Conversely, thermal energy can also be converted into kinetic energy. For instance, in a heat engine, thermal energy from a fuel source is converted into kinetic energy to power the engine.
Quick Check
Comparison of Kinetic and Thermal Energy
| Characteristic | Kinetic Energy | Thermal Energy |
|---|---|---|
| Definition | Energy of motion | Energy due to random particle motion |
| Measurement | Joules | Joules |
| Factors | Mass, speed | Temperature |
| Transfer | N/A | Conduction, convection, radiation |
Common Misconceptions
- Kinetic energy is only possessed by moving objects.
- Thermal energy is only present in hot objects.
- Kinetic energy and thermal energy are not interchangeable.
Practice Problems
- Calculate the kinetic energy of a 10 kg object moving at 5 m/s.
- Explain how thermal energy is transferred from a hot stove to a pot of water.
- Describe a real-world application where kinetic energy is converted into thermal energy.
FAQ Compilation
What is the primary difference between kinetic and thermal energy?
Kinetic energy is associated with the motion of an object, while thermal energy is associated with the temperature of an object or system.
Can kinetic energy be converted into thermal energy?
Yes, kinetic energy can be converted into thermal energy through processes such as friction and collisions.
What is an example of thermal energy transfer by conduction?
When you touch a hot stove, heat from the stove is transferred to your hand through conduction.
